Introduction
Milk fats consist of a mixture of compounds including triacylglycerols, diacylglycerols, monoacylglycerols, phospholipids, cerebrosides, gangliosides, sterols and sterol esters and derivatives, carotenoids, tocopherol, vitamins A,
D, E, C, B1, and B2 and free fatty acids. They are not soluble in water, or aqueous liquids.
Milk fat is secreted from the mammary epithelial cells as fat globules, the average size of a milk fat globule is about 3um in diameter, but they can range from 1 to 18 um.
The milk fat globule is encapsulated by a lipid bilayer membrane made up of proteins, phospholipids, lipoproteins, cerebrosides, nucleic acids, enzymes, trace elements, and bound water.
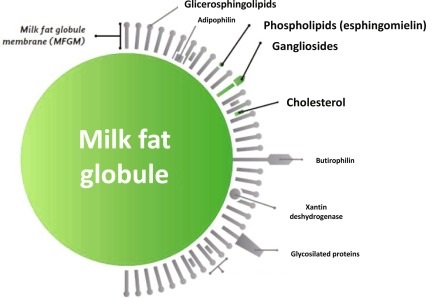
The FGM (fat globule membrane) provides stabilization for the fat globule in the aqueous environment of the milk serum. When the FGM membrane is ruptured, the fat globules join together into a solid mass of fat. (This is what happens during the production of butter).
Milk fat globules are the largest particles in milk but they are also the lightest, They have a lower density than milk serum, so the globules rise to the surface, which is how cream is formed. The density difference is used to separate milk fat from whole milk in high speed, continuous flow, centrifugal separators.
All fats belong to a group of chemical substances called esters, which are compounds of alcohols and acids. Milk fat is a mixture of different fatty acid esters called triglycerides.
Triglycerides (triacylglycerols) account for 97-98% of the total fat in cows’ milk. The remaining lipid classes are di- and mono-acylglycerols, phospholipids, free fatty acids, and cholesterol and its esters.
A triglyceride contains a glycerol backbone. Free fatty acids are attached to the glycerol backbone by an ester bond. Each glycerol molecule can bond three fatty acid molecules. About 437 fatty acids have been identified in the milk fat of cows.
These fatty acids all vary in chain length, number, position, and geometric isomerization of double bonds (cis or trans).
A fatty acid molecule is composed of a hydrocarbon chain and a carboxyl group. In saturated fatty acids, the carbon atoms are linked together in a chain by single bonds. In unsaturated fatty acids, there are one or more double bonds in the hydrocarbon chain.
Synthesis of Milk Fat in Cow Milk
Fatty acids in milk come from three main sources:
from the feed, from the mobilization of reserve tissue, and from de novo (from the beginning) synthesis within the cow.
The main source of lipid is from the cow’s food. Little synthesis of fatty acids occurs in the mammary gland. When the cow consumes food, the lipid is hydrolyzed to free fatty acids within the rumen of the cow. This means that unsaturated fatty acids are usually hydrogenated into saturated fatty acids. Hydrogenation is the addition of hydrogen on unsaturated bonds between
carbon atoms.
Although it seems that milk fat would be mostly saturated in nature, over 30% of milk fat is still unsaturated. The unsaturated milk fat is predominately oleic acid (C18:1). Stearic acid (a saturated fatty acid) enters the mammary gland, but within the gland there is a specific C18:0 desaturase which converts stearic acid to the unsaturated oleic acid. As a result, there is a significant amount of unsaturated fat in milk.
Just know that a desaturase is an enzyme which removes two hydrogen atoms from the fatty acid, creating a double
bond.
From the rumen, the fatty acids have two places they can go: 1. the bloodstream to be deposited as reserve fat or to be metabolized to produce energy, or 2. the mammary gland.
Distribution of fatty acids
The characteristics of milk fat are highly dependent on the nature of the pre-formed fatty acids available in feedstuff. Therefore, it is possible to manipulate the composition of milk fat by dietary means.
Milk fat of cows can be separated into long-chain and short-chain fatty acids fractions by distillation or chromatography. The short chain fraction contains mostly butyrate.
The physical properties of butterfat can be controlled by dietary means because the melting properties of the triglyceride are related to fatty acid composition. For example, by dietary manipulation, butter which can be spread directly from the refrigerator can be produced. On the other hand, butter that is physically stable at high temperatures can also be made.
During colder winter months, there are more pre-formed fatty acids in the cows diet than during the summer months. During the summer, cows graze on grass and drink more water than in the winter months.
Milk fat is characterized by the presence of relatively large amounts of butyric and caproic acid, the four most abundant fatty acids in milk are myristic, palmitic, stearic, and oleic acids. Myristic, palmitic, and stearic acids are solids at room temperature and oleic acid is a liquid at room temperature.
The amounts of fatty acids in milk are variable. This variation affects the hardness of fat. Fat with a high content of high-melting fatty acids, such as palmitic acid, will be hard. However, fat with a high-content of low-melting oleic
acid makes soft butter.
Difference between the milk fat of humans and cows.
The milk fat from the cow contains a set of unique short carbon chain fatty acids (4:0 – 10:0) and very little polyunsaturated fatty acids (18:2+3). These differences are a result of the digestive system of the cow. The cow has a rumen, which you all may already know about. It is basically a large fermentation tank in which food is broken down and modified by microorganisms before it goes into the intestines.
The abundant polyunsaturated lipids in the grasses, legumes, silages, and grains consumed by the cow are hydrogenated (saturated) in the rumen, which is why cows milk has saturated fats. Acetic and beta-hydroxybutyric are products of fermentation in the rumen and they are absorbed directly from the rumen into circulation and are carried to the mammary gland where they are used to make short chain fatty acids.
AS per the study when the human mother was fed corn oil, which is rich in linoleic acid (18:2),
she produced a level of 43% of linoleic acid in her milk fat.
Changes in milk fat during storage
Oxidation of milk fat
Oxidation occurs at the double bonds of the unsaturated fatty acids. The presence of iron and copper salts accelerates the onset of auto-oxidation and the development of a metallic flavor.
Also, the presence of dissolved oxygen and exposure to light promotes oxidation. Oxidation of milk fat may limit the
shelf life of whole milk powders, but is not usually a serious problem.
Oxidation is catalyzed by light, so if milk is exposed to light for too long a period of time, off-flavors will occur. Fat oxidation can be counteracted by microorganisms in milk such as lactic acid bacteria because they consume oxygen thereby
reducing oxidation.
Also, pasteurization helps to reduce oxidation because reducing compounds such as sulfhydryl groups are formed when milk Is heated.
The metallic oxidation off-flavor is more common in the winter than in the summer because of differences in the cows diet. Summer feeds are higher in Vitamins A and C. These vitamins increase the amount of reducing substances in milk.
Hydrolysis
Hydrolysis is the liberation of free fatty acids from the glycerol backbone. This reaction requires the presence of a lipase, which is an enzyme that catalyzes he hydrolysis of ester bonds of lipids. A large amount lipoprotein lipase is present naturally in milk, but fortunately fat globules with an intact milk fat globule membrane are not susceptible to hydrolysis by the enzyme. Also, lipoprotein lipase is inactivated at temperatures required for pasteurization.
Spoilage bacteria provide a heat stable lipase, but the spoilage bacteria must exceed normal levels, and routine bacterial control should prevent such an occurrence.
Lipolysis
The breakdown of fat into glycerol and free fatty acids is called lipolysis. Fat that has been lipolysed tastes rancid and smells rancid. This effect is due to the presence of low molecular free fatty acids such as butyric and caproic acids.
Lipolysis is enhanced by lipases and high storage temperatures. Lipases can only act if the fat globules have been damaged so that the fat is exposed. The fat globules can be damaged by pumping, stirring, or splashing the milk. Therefore, unnecessary agitation of unpasteurized milk should be avoided to prevent the damage of the fat globules.
High temperature pasteurization inactivates the lipases.