Introduction
There are two distinct types of milk proteins, casein and whey. Caseins make up over 80% of the total
milk protein content and they can be further divided into five groups – the alpha s1, alpha s2, beta, gamma, and
kappa caseins.

Caseins- Milk Proteins
Caseins do not have an organized structure, thus they cannot be denatured by heating. The casein molecules form polymers containing several identical and different molecules. Some molecules have hydrophillic regions and some have hydrophobic regions.
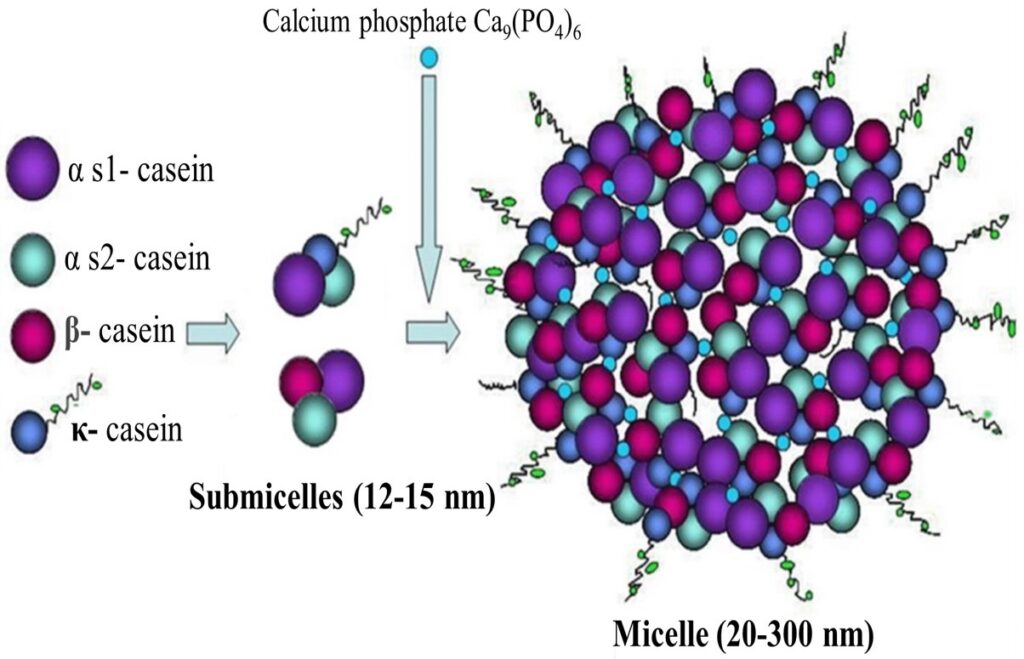
The polymers are made up of many individual molecules and form into casein micelles. They are also formed into a colloidal solution, which gives skim milk its whitish-blue color.
The alpha and beta caseins account for the calcium sensitivity of milk protein. Casein coagulates when calcium is added to casein solutions. This reaction is the key to cheese making and to the formation of acidic gels such as yogurt. Casein also coagulates when rennet is added. Rennet is a proteolytic enzyme that is found in the stomachs of calves.
The amino acids in casein have hydrophobic and hydrophilic regions, allowing the caseins to act as highly effective surface active agents. In other words, caseins act as stabilizers of foams and emulsions.
If an acid is added to milk or if acid producing bacteria are allowed to grow in milk, the casein micelle will change in two ways. First, colloidal calcium hydroxy-phosphate, present in the casein micelle, will dissolve and form ionized calcium. This ionized calcium will penetrate the micelle structure and create strong internal calcium bonds.
Second, the pH of the solution will approach the isoelectric points of the individual casein molecules. So you’re probably thinking great – what does this mean? What happens is this – the casein micelles get
bigger because they aggregate with one another.
The making of cottage cheese is a great example of this principle. Also, if one can I tried to make a lower
fat lemony alfreado sauce using 2% milk instead of heavy cream but the lemon lowered the pH of the milk and the casein in the milk coagulated and made cottage cheese instead!
Whey
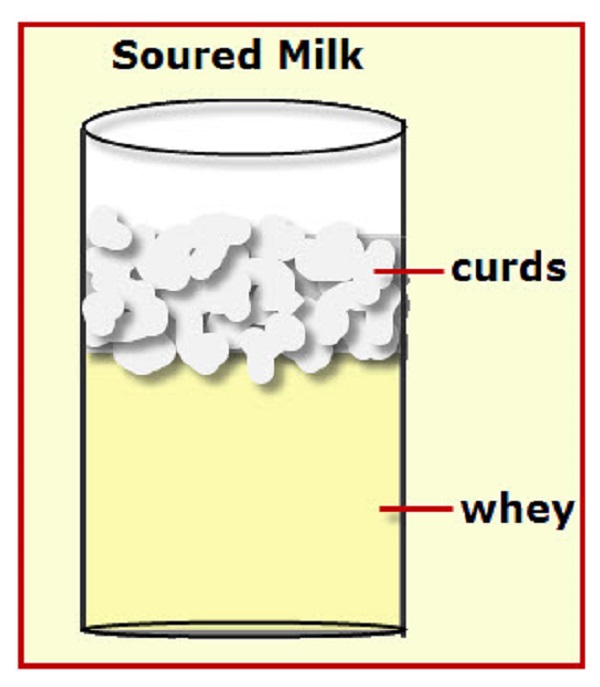
Whey is the milk serum proteins. When casein is precipitated out of skim milk, whey is left over. Casein are the curds and the liquid left over is the whey.
Whey proteins are globular proteins which may be denatured when heated above temperature above 65 deg. C. The major components of whey are beta lactoglobulin and alpha lactalbumin. Whey is the liquid remaining after milk has been curdled and strained during the manufacture of cheese.
It is used to produce ricotta and brown cheeses and is an additive in many processed foods, such as breads,
crackers, pastries, and animal feed.
Many of the important nutrients that are comprise milk are partitioned into whey. It contains many important nutrients, such as lactose, water soluble vitamins, and most of the minerals, with the exceptions of considerable calcium and phosphorus which go with the casein into the cheese curd.
The amino acid composition of whey is very close to that which is considered as the optimum amino acid
value.
Alpha-lactalbumin-
Alpha-lactalbumin is considered the typical whey protein. It is present in milk from all mammals and it plays an important role in the synthesis of lactose in the udder.
Beta-lactalbumin –
This protein is found only in ungulates and is the major whey protein component of milk from cows.
Milk Proteins – Reactions
The most important reactions of the milk proteins are those which involve destabilization of the casein micelles.
As we previously mentioned, reactions can occur within casein micelles as the pH is reduced. As the pH of milk is reduced, the native micellar structure disintegrates. At the isoelectric point of casein, (the pH at which casein carries no net electrical charge – a pH of 4.6), the casein precipitates out of solution, along with any heat-denatured whey.
This reaction may be reversed as the pH is increased, but native form of the micelle is not recovered.
Acidification of milk may be used to form many fermented products such as yogurt and to fractionate the milk proteins to produce acid casein. Acid casein can be purified and used in foods for its emulsifying and water binding properties.
At a neutral pH, milk is very chemically stable. However, when milk is heated, added to alcohol, or concentrated, stability may be reduced.
Destabilization of casein micelles also occurs when milk is subjected to selective proteolysis. In selective proteolysis, a specific enzyme, chymosin, cleaves off the part that stabilizes the kappa casein. This proteolysis results in an increase in sensitivity of the casein micelles to calcium, so the two coagulate and a firm gel is formed.
These reactions form the basis of cheese production. However, this process is actually made up of two reactions: proteolysis and calcium-induced aggregation. If the reaction is carried out at refrigeration temperatures, only proteolysis occurs and the milk remains stable until heating takes place and the calcium-
induced clotting can occur.